The Story
A young woman loses consciousness at home and is unable to be revived. EMS brings her to the hospital with resuscitation in progress.Initial ECG:
Initial labs:
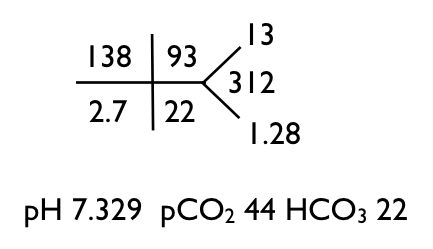
What's the acid base disorder?
I look at the increased pCO2 and decreased pH and call it a respiratory acidosis. The HCO3 should go up to compensate for the increased CO2 but it is slightly decreased, so there is also a metabolic acidosis.
When there is a metabolic acidosis check the anion gap (and even when there is no evidence for a metabolic acidosis, you should check for an anion gap). Holy Ketones, the anion gap is 23!
A large anion gap in the face of only a modest drop in bicarb is unexpected. One expects the bicarbonate to fall 1 mEq/L for every 1 mEq/L the anion gap rises. So if this lady started with an anion gap of 12, her gap went up by 11, so the bicarb should have fallen by 11. In order for the bicarbonate to fall by 11 and end up at 22 it would need to start at 33, indicating a preexisting metabolic alkalosis (or compensated respiratory acidosis I suppose). The metabolic alkalosis is welcome because it fits with the hypokalemia rather well. Alaklosis and hypokalemia go together like macaroni and cheese.
So what is causing the increased anion gap? Well that is easy to determine in this case, her lactate was 12.4, almost a perfect match for an increase in the anion gap of 11. Remember the anion gap is quantifying an anion that is not in the chem-7 but is making its presence known by the unbalanced number of cations and anions. We expect the unbalance to be between 6 and 12, this is a normal anion gap. Her anion gap is 23, 11 over the limit. This means we need to find the anion that explains this gap of 11. A lactate level of 12.4 neatly explains the increased gap. If you are concerned about the difference between 11 and 12, remember that the calculation of 11 comes from assuming that prior to building up the lactic acid, her anion gap was 12, but it could have been 8 or 10 or 4.
What happens next will shock you (now I'm a real internet writer)
The potassium went down. It went way down...to 1.8, and that, my internet friends, is the lowest potassium I have ever seen. And it is the presumed cause of the cardiac arrest. The ECG normalized with correction of the potassium.
For the next 38 hours we give her potassium. A lot of potassium. Especially if you consider that she was anuric due to acute tubular necrosis.
Keep in mind that the entire extracellular compartment normally contains only 56 mmol of potassium. The entire plasma compartment normally contains only 12 mmol of potassium. Also keep in mind that after 810 mmol of potassium she was still hypokalemic.
So what happened after 38 hours?
We overshot, in fact we made her hyperkalemic and needed to start dialysis. The dialysis was inevitable, she remained anuric for days after we started the CRRT, and she was also becoming volume overloaded.
The cause of the hypokalemia is unclear. The one point that I am certain of, though, is this must be a chronic process. She had a documented potassium deficiency of 31 grams of potassium! No acute event could have caused her to lose that much potassium, the process that caused the hypokalemia and metabolic alkalosis must have been going on for weeks? Months? Years? Prior to her developing the cardiac arrhythmia. She has recovered her renal function and her electrolytes remain within normal limits, she does not seem to have any renal leak of potassium that I can detect. She denies vomiting. She does have a poorly documented incident of hypokalemia that happened years ago. Her magnesium when measured on the first hospital day was normal. Since recovering her kidney function her magnesium has been low normal. She denies salt-cravings. Just after she recovered her kidney function and her creatinine was normal, she developed slight hypokalemia, right around 3.2 mmol/L. I shot off a TTKG and it was an unremarkable 3.1.
So smart people on the internet, what do you think caused the hypokalemia. The family is understandably concerned that this could happen again. I have my theory, but I don't want to bias your thoughts. Please e-mail me or DM me on Twitter.