There was an interesting and in depth discussion of the issues on Twitter. I captured the major points this Storify:
Tuesday, December 17, 2013
Vanco Pip/Tazo and AKI
On Sunday the story broke that a single center study had found increased AKI with the combination of Vancomycin and Pipercillin-Tazobactam.
There was an interesting and in depth discussion of the issues on Twitter. I captured the major points this Storify:
There was an interesting and in depth discussion of the issues on Twitter. I captured the major points this Storify:
Wednesday, December 11, 2013
Another Candidate for Top Nephrology Story of 2013: HDPAL
Another guest post, this by Christos Argyropoulos of Athens. You know him on Twitter as @ChristosArgyrop.
Lisinopril vs. Atenolol for hypertension in the dialysis unit
Background and Rationale
A study reporting the relative effectiveness of lisinopril over atenolol for hypertension in the hemodialysis unit was presented in the 2013 Kidney Week meeting. The HDPAL (NCT00582114) was a parallel group, open label randomized control study conducted between 2005-2013 in a single center that was sponsored by IU and supported by NIDDK. The aim of HDPAL (per the clinicialtrials.gov entry) was to “directly test the hypothesis that an initial strategy of lisinopril-based therapy will be more effective than atenolol-based therapy in causing regression of LVH over one year in patients with hemodialysis hypertension despite similar degree of BP reduction”. The justification for the study was provided by the high prevalence (>80%) of hypertension in the dialysis unit and the associated morbidity of stroke and left ventricular hypertrophy, while the choice of interventions tested in HDPAL was motivated by prior pilot studies performed by the PI in the late 1990s. These studies showed that atelolol and lisinopril are both able to reduce monitored ambulatory blood pressure (ABPM) to a similar extent i.e. by ~20/11 when dosed three days a week after dialysis in a supervised fashion.
Study Procedures and Outcomes
HDPAL randomized HD patients with echocardiographically documented LVH to either atenolol (initial dose: 25mg TIW, titrated to a maximum of 100 mg by doubling the dose q2wks) or lisinopril (initial dose: 10 mg TIW titrated to 40 mg by doubling the dose q2wk). To be eligible to participate, patients with LVH had to either have an ABPM > 135/75 (after a UF trial), or not be receiving any antihypertensives if they did not want to participate in a UF trial. Felodipine (10 mg) could be added once the maximum dose of the study medications had been reached and other anti-hypertensive agents could be added after felodipine if ABPM did not decline below 140/90.
The primary outcome for the study was regression of LVH by echocardiographic criteria at 12 months, while secondary outcomes to be assessed were regression of LVH by echo at six months and the adjusted (for age, gender, ABPM) index of LV mass/BSA from baseline to month 12.
Patients would have echocardiographic assessments at baseline and again at 6 and 12 months, as well as ABPM at baseline and 3, 6, and 12 months and were also asked to self monitor their BP.
What did the study show?
The study enrolled 200 (mostly African American) patients, but it was stopped prematurely for safety reasons so that only 104 patients completed follow-up (58 in the atenolol and 46 in the lisinopril arm). At the time the study was stopped, patients on atenolol had numerically higher reductions in LVH but this finding did not reach statistical significance. The safety signals were ubiquitous for patients on lisinopril:
- Excess number of serious cardiovascular events(IRR: 2.36, 95% CI 1.36 to 4.23, P=0.001)
- Excess major cardiovascular events (IRR 2.29 95%CI: 1.07-5.21 p=0.02)
- More frequent hospitalization for all causes (IRR: 1.61 p<0 .01="" li="">
- More frequent hospitalization for congestive heart failure (IRR 3.13 p=0.02)
- More likely to develop hyperkalemia (IRR 3.38 p=0.05)
- More likely to suffer a hypertensive crisis (IRR 3.81 p=0.03)
On the other hand, atenolol was more efficacious in reducing BP, irrespective of the method of assessment (ambulatory v.s. home self-monitoring) by 3.5 and 6.3 mmHg respectively). These patterns of suboptimal BP control by lisinopril were noted despite more aggressive fluid removal and a larger number of additional blood pressure medications.
Discussion
As the study was terminated prematurely, the primary and secondary end points could not be fully assessed and the superiority of beta blockers versus ACE inhibitors remains an open question. On the other hand, the reported patterns of blood pressure reduction and the cardiovascular safety signal were surprising given the established track record of ACEis in reversing LVH and the poor relative efficacy of atenolol against other agents in the non-ESRD population (Cochrane Database Systemic Review, though not everyone agrees with this interpretation e.g. Blood Pressure, 2007 and BMJ, 2009). However tempting it may be to attribute these findings to a play of chance, or undifferentiated secular trends (e.g. the study was registered with Clinical Trials.gov in 2005, yet only 104 patients had a year of follow-up by 2013) the possibility that atenolol is indeed a better drug than lisinopril for dialysis patients with LVH should be entertained. Working under this hypothesis, there are at least two possible explanations for the apparent benefit of atenolol on blood pressure control:
- A ghost of studies past: A number of studies have suggested that African American patients with cardiac disease (either LVH or systolic dysfunction) may respond better to beta blockade compared to RAAS inhibition (a pattern seen in the Losartan Intervention for Endpoint Reduction – LIFE study), or even receive no benefit from ACEs compared to whites (e.g. SOLVD). Considering that the mostly African American participants in HDPAL were on the ultimate “diuretic” (dialysis to dry weight), an intervention that diminishes the relative (in)effectiveness of beta blockers in the non-ESRD African American population, one could hypothesize that the results of this study may reflect a racial benefit of beta-blockers for African American with LVH. It would be interesting to see whether the investigators of the HPDAL broke down the results according to race, as a hypothesis generating analysis.
- Pharmacokinetics: Atenolol and lisinopril pharmacokinetics on dialysis differ and these differences may explain the inferior blood pressure control in home and ABPM recordings. While both agents are efficiently cleared by hemodialysis, with apparent intradialytic half life between 3.5-5 hrs (atenolol: BJCP, 1980, Arch Toxicol Suppl, 1980 and Eur J Clin Pharmacol, 1981, Hemodialysis International 2013 and lisinopril BJCP, 1988) there are important differences in the time to the peak (~4hrs with atenolol, 8-44 hrs with lisinopril) and rebound kinetics (larger rebound with atenolol). Based on these considerations one could hypothesize that patients receiving atenolol in the dialysis unit would spend a much shorter period of time under-medicated as a result of the faster absorption of the drug and possibly its higher rebound. This could explain the larger time difference in BP noted in ABPM recordings and home BP measurements. To the extent the investigators obtained blood levels, it might be possible to explore the pharmacokinetic hypothesis by correlating free drug concentrations to ABPM or home BP recordings. To the extent that pharmacokinetics play a role in explaining the HDPAL results, one could consider using alternative RAAS inhibitors (e.g ramipril) that are exhibit more comparable kinetic behavior to atenolol.
Implications for clinical practice
This is an interesting pilot report about therapeutic intervention to control hypertension in dialysis, a common problem for our patients. Current approaches to this problem are unsatisfactory, judging from the frequency with which nephrologists switch agents (BMC Nephrol. 2013) in the unit. Far from definitively proving the superiority of beta blockers over ACE inhibitors due to the limitations of a prematurely stopped and thus underpowered study, HDPAL adds some important information that could help clinicians choose blood pressure medications for their dialysis patients. In particular, HDPAL suggests that the perceived inferiority of beta blockers in the non-ESRD population may not apply to dialysis patients. Though it is customary to say that further studies will be needed (and in fact we do need them!), clinicians managing hypertensive dialysis patients should lead by example and consider applying the HDPAL protocol in the context of “n=1” trials. These studies “consider an individual patient as the sole unit of observation” to investigate the efficacy or side-effect profiles of different interventions (Per Med. 2011). In particular, rather than switching antihypertensives around in no systematic pattern, the nephrologist working with the patient under a shared decision making paradigm carry a structured evaluation of lisinopril (the most commonly prescribed ACEi, used in 20.9% of dialysis patients) vs. atenolol (the least prescribed beta blocker, but still used in 7.2% of patients) correlating home blood pressure (or ABPM if available) in a 4 period crossover fashion. By pooling multiple such studies it may be possible to fill in the knowledge gap that HDPAL tried to fill.
Tuesday, December 10, 2013
Top Nephrology Stories of 2013 NEPHRON-D: The End of the Combination Treatment Era
 This is a guest post. I don't think I have ever done this before but we are trying to get as many voices to weigh in on the Top Nephrology Stories of the Year as possible and I knew that I wanted Ed El Sayed (@iApothecary) to be part of this. If you don't follow him, you should, he is one of the sharpest medical minds on Twitter. When I invited him he said he had no place publish, so I offered a temporary home on PBFluids. Hey Ed, start a Tumblr, they're free!
This is a guest post. I don't think I have ever done this before but we are trying to get as many voices to weigh in on the Top Nephrology Stories of the Year as possible and I knew that I wanted Ed El Sayed (@iApothecary) to be part of this. If you don't follow him, you should, he is one of the sharpest medical minds on Twitter. When I invited him he said he had no place publish, so I offered a temporary home on PBFluids. Hey Ed, start a Tumblr, they're free!Here are his words on the top nephrology story of the year:
 Diabetic nephropathy is the leading cause of Chronic Kidney Disease (CKD) and End Stage Renal Disease (ESRD) in the US. It has long been postulated that combination pharmacotherapy with
Diabetic nephropathy is the leading cause of Chronic Kidney Disease (CKD) and End Stage Renal Disease (ESRD) in the US. It has long been postulated that combination pharmacotherapy withAngiotensin Converting Enzyme Inhibitors (ACEi) and Angiotensin Receptor Blockers (ARBs) could slow the progression of nephropathy, and have a positive impact on the prognosis, morbidity, and mortality of patients with diabetic kidney disease. This hypothesis arose from the unfortunate fact that neither ACEi nor ARBs working alone are able to fully block the renin angiotensin aldosterone system (RAAS).
Design of Combination Angiotensin Converting Enzyme Inhibitor Angiotensin Receptor Blocker for Treatment of Diabetic Nephropathy (NEPHRON-D) was a large multi-center, prospective, and parallel trial that was terminated early due to serious side effects. In this study, 1,648 patients (average age of 64 years) with type 2 diabetes, albuminuria, and stage 3 or 4 CKD were recruited. All patients were started on losartan 100 mg and then randomized to receive either Lisinopril 10-40 mg daily or placebo..
As mentioned above, this well designed and well powered trial was terminated early due to a significantly higher rate of adverse events with combination therapy. Investigators reported that the incidence of hyperkalemia was higher with ACEi and ARBs compared to ARBs and placebo (6.3 vs 2.6 events per 100-persons-years with P = 0.001). Acute Kidney Injury (AKI) was also higher with combination therapy (12.2 vs 6.7 events per 100 person-years with P = 0.001).
With 2.2 years of follow up investigators reported no significant difference in the study’s primary end point of progression of renal disease or death (152 vs 132 with P = 0.3).
The ONTARGET and ALTITUDE studies were the two previous attempts to show renal or cardiovascular benefits from combination RAAS blockade (ACEi + ARBs in ONTARGET; ACEi + Renin Inhibitor in ALTITUDE). ONTARGET involved patients with an increased risk for cardiovascular disease but predominantly normal albumin excretion levels, while ALTITUDE involved patients with kidney disease and diabetes who were not necessarily proteinuric. Neither showed any benefit and had increased adverse events with combination therapy.
Experts now have three solid trials that clearly convey one message: Combination pharmacotherapy with ACEi and ARBs in patients with DM induced nephropathy and CKD does not provide any additional benefit and may, in fact, increase the incidence of harm.
It is worthy to note that NEPHRON-D is a perfect example of a “negative” trial that has a huge impact on the strategies used by clinicians in treating their patients; emphasizing the importance of publishing both negative and positive outcome trials.
Update from Twitter:
@kidney_boy IIRC COOPERATE was retracted due to fabricated data, so that only one name should be engraved on that stone
— ChristosArgyropoulos (@ChristosArgyrop) December 11, 2013
Nice discussion about how COOPERATE was retracted via @cardiobrief http://t.co/nEbs4P2cS9
— Matt Sparks (@Nephro_Sparks) December 11, 2013
I set the onset of combo therapy at the publication of COOPERATE because it was a landmark study at the time. It was regularly brought out as justification for the use of dual therapy, because it's end-point was doubling of serum creatinine or ESRD, not just a change in proteinuria. According to the Cardiobrief pos,t that Dr. Sparks referenced, combo therapy was already in wide use at the time of publication but I think COOPERATE was the study that made its use acceptable to EBM purists.
Monday, December 9, 2013
I get some great letters, here is one of the best from a woman with SIADH
An e-mail I received last month:
I love your blog. I have had SIADH for a zillion years. I only found out what I had when I went with 5 girlfriends to a fancy spa hotel in Tucson for a mini-vacation/ 4th of July Weekend in 1997 where the heat increased to an uncomfortable 117 degrees.
Healthcare workers in the hotel kept handing out bottles of water at each hotel exercise location with orders to “hydrate, hydrate, hydrate” and I stupidly followed their directions. I drank myself into a 6 day coma.
Very non-traumatic for me. Very traumatic for my family. I woke up on day 6 saying, “I am STARVING! Will someone go get me a taco?” which was very anxiety-relieving for all of them; they’d been sure I’d wake up cognitively impaired. I wasn’t. This “taco” sentence sounded JUST like me. And I have continued to be not cognitively impaired despite interesting lab numbers.
My dad (who is a physician too) has SIADH as well, though his was diagnosed after mine. I was mis-diagnosed for 9 years prior to my coma as having a “seizure disorder.” The excellent care I received when my mental status went to heck in a handbasket was truly life-saving. I remain a very grateful nephrology patient. And I really do love your blog.
I love your blog. I have had SIADH for a zillion years. I only found out what I had when I went with 5 girlfriends to a fancy spa hotel in Tucson for a mini-vacation/ 4th of July Weekend in 1997 where the heat increased to an uncomfortable 117 degrees.
Healthcare workers in the hotel kept handing out bottles of water at each hotel exercise location with orders to “hydrate, hydrate, hydrate” and I stupidly followed their directions. I drank myself into a 6 day coma.
 |
| The only time that sentence has been used for water, not alcohol. |
My dad (who is a physician too) has SIADH as well, though his was diagnosed after mine. I was mis-diagnosed for 9 years prior to my coma as having a “seizure disorder.” The excellent care I received when my mental status went to heck in a handbasket was truly life-saving. I remain a very grateful nephrology patient. And I really do love your blog.
Thought you should know this.
She wrote back a few days later giving me permission to post her letter:
I am dying (well not literally dying) to start an SIADH group on Facebook. We are so not connected to one another, and each of our nephrologists only have a handful of patients and of course the doctors can't introduce us to eachother because of HIPAA.
- For those of us that have the Syndrome without lung cancer and so on and have to live our lives thirsty
- and our summers avoiding the sun through our sunroofs (Demeclocycline)
- and have to, if we're female, find inventive ways to paint our nails to avoid Demeclocycline making our nailbeds ugly colors
- and have to fear that Otsuka Pharmaceutical will convince the ONE manufacturer who makes Demeclocycline to stop making it and force us into buying Tolvaptan even though they never tested it in 3rd stage human trials on people that weren't already cognitively impaired (I know because I volunteered for every single US trial),
Well, we NEED each other. We need tips on nail polish, tips on drinking our fluids out of 1 ounce shot glasses, tips on rolling ice cubes around our mouths during the days our sodium is tanking, and your website is a GREAT place for us to meet up!
You have my non-dying thanks and permission to reprint/repost any or all of my statements!
The best story that wasn't nominated.
Its oscar season in nephrology. The RFN is winnowing down the top stories in nephrology of 2013 but maybe the most important isn't even nominated. It came out too late. On November 27, Kidney International released this study in advance of print:
The bullet point I learned in fellowship was that the rate of renal failure in kidney donors was no higher than the general public, of course the kidney donors are screened to be much healthier than the general public, so the fact that the rate of renal failure is not significantly lower than the general public is an important signal.
Donors don't have obesity, diabetes, hypertension. Of course most of them also have family history of kidney disease, so when they do develop kidney failure attributing the cause cause can be tricky, certainly some of these people would progress to renal failure even if they didn't donate.
Into this statistical Gordian knot wades the Norwegians.
The bullet point I learned in fellowship was that the rate of renal failure in kidney donors was no higher than the general public, of course the kidney donors are screened to be much healthier than the general public, so the fact that the rate of renal failure is not significantly lower than the general public is an important signal.
 |
| Have I mentioned how much I love MedCalc? |
Into this statistical Gordian knot wades the Norwegians.
- 15 years of follow up for donors that were 46 years old at the time of donation. That is adequate follow-up in my mind.
- Their center had no preoperative mortality. They did 2,269 living donations with out losing a donor. Great work by the surgical team.
- They found an increased risk of death by any cause, HR 2.49 (CI 2.13-2.91).
- Adjusted HR for all-cause mortality fell to 1.48 (CI 1.17-1.88).
- I used their raw mortality data to calculate the Number Needed to Harm. It was a frightening 23 (4.3% absolute risk increase). I do not think this is a valid use of NNH, see below.
- The CV Mortality HR was 1.4 (CI 1.03-1.91).
- ESRD was increased from 0.01% per year to 0.03% a relative risk of 3 but a number needed to harm of 5000. (i.e. you would have to do 5,000 living transplants a year to see one additional cases of dialysis in a donor)
 |
| The lines only begin to separate after 10 years. This indicates that we should probably ignore studies with less than a decade of follow-up |
The data is eye opening, but I would really be interested in seeing what the difference between first degree relatives and people not related. I think much of the increased danger comes from being related to the patient who needed the transplant in the first place.
I also recommend ignoring all the unadjusted data (including the number needed to harm) because the control group was nearly a decade younger than the donors. A ten year difference in age when the total follow-up is only 16 years makes the unadjusted data deceptive, IMHO.
I would love to see a study where the control group was made up of other people who were evaluated and cleared to donate a kidney but ended up deferring, due to the recipient getting another organ or dying or going off the list for some other reason. That would be the ideal control population.
The reality is that people want to donate a kidney to their loved ones. Telling them there maybe a small increase risk of death when you are in your late 50's or early sixty's. Will probably not eliminate many close relatives, but altruistic donors and family members should be aware of the risks as understood today. It is probably time to bury the old line about "No increased risk of renal failure than the general public."
Another study on this from 2010 can be read here.
Another study on this from 2010 can be read here.
Wednesday, December 4, 2013
Add to the list of top stories of 2013
In my manifesto on Tolvaptan, I mentioned there was another breakthrough that should be on the list of top stories of 2013, and it is...The gathering momentum of renal denervation for hypertension. This feels like it is rapidly maturing and will be a viable option in the near future.
The latest data presented at American Heart Association looks promising.
Go to Renal Fellow Network. Vote for your favorite!
The latest data presented at American Heart Association looks promising.
Go to Renal Fellow Network. Vote for your favorite!
Sunday, December 1, 2013
Go #TeamTolvaptan
 |
| This is just a picture of the ballot go to RFN to cast you ballot. |
(I thought of one more but it escapes me, I'll update this post when I remember it.)
Looking over the list, I think the story of the year is Tolvaptan's failure to FDA gain approval for ADPKD. If Tolvaptan gains FDA approval in the next year or two following re-submission this story will quickly be forgotten. But if the future goes the other way, if Otsuka abandons tolvaptan for ADPKD and decides to remain in the limited ghetto of inpatient management of hyponatremia that would be a tragedy.
Part of what gets my blood boiling is the raging hypocrisy that the very same organization that approved:
- Zemplar (paricalcitol)
- Hecterol (doxercalciferol)
- Renagel (sevelamer)
- Renvela (sevelamer again)
- Fosrenal (lanthium carbonate)
- Sensipar (cinacalcet)
(all drugs that have never been shown to help a patient but merely to improve some biochemical target. Hell, even sevelamer and cinacalcet tried and failed to show reduced mortality and they remain on the market) had the gall to deny tolvaptan for ADPKD.
ADPKD slowed cyst growth and slowed the progression of kidney disease. Not just for one year but for three years! It joins ACEi/ARBs and insulin in the pantheon of drugs that can slow the progression of CKD (though bicarbonate and allopurionol are walking toward the door). Denying it was preposterous.
ADPKD slowed cyst growth and slowed the progression of kidney disease. Not just for one year but for three years! It joins ACEi/ARBs and insulin in the pantheon of drugs that can slow the progression of CKD (though bicarbonate and allopurionol are walking toward the door). Denying it was preposterous.
The other part of my rage comes from possibilities of having tolvaptan available as an outpatient option for patients. I certainly want this drug available for ADPKD but turning it from a limited duration in-patient drug to a chronic outpatient therapy will completely remake the cost structure.
The price will remain high but it will no longer be $300/pill. Let us say $1000 per month. The TEMPO trial used 120 mg per day, so a month worth would be 120 pills. That would go a long way if you were interested in using tolvaptan for aquaresis. This drug is effective at increasing urine output by a mechanism totally novel to us. We are being given control over one of the fundamental control levers of the kidney. There are only limited number of these levers:
Look it how useful the drugs are that affect these hormones. The list is full of all-stars. Tolvaptan has already been proven a winner in ADPKD and maybe useful in CHF. Tolvaptan failed a pivotal trial called EVEREST, but the drug did increase diuresis, fluid loss, and the effects were long lasting. Tolvaptan failed one trial on heart failure, but alternative patient selection or dosing regimens could turn this turkey into another critical tool for heart failure. We need to have this drug available to see where it can be useful because today we are primitives.
If the FDA approves it, the fact that it failed its first application will be quickly forgotten, but if Otsuka decides to retreat from this breakthrough and live happily ever after correcting mild asymptomatic hyponatremia nephrology will be a poorer place.
Monday, November 25, 2013
Impact factor for blogs
For people unaware of impact factor, it is the AP Top 25 for scientific journals. It provides a score for every journal based on how often scientific articles cite articles published by the journal. The number of citations is divided by the number of articles published so just pushing a lot of crap through in hopes that some of it gets cited does not inflate your score. The top three medical journals are NEJM, Lancet and JAMA in that order. Journal editors spend a lot of time worrying, strategizing and optimizing in order to improve Impact Factor.
I had the privilege of going to the AJKD editors meeting Kidney Week and saw first hand how IF colors a lot of what they do. Editor in chief, Dr. Levey talked about change in IF over the last decade and what happened over the last year. Then he talked about the different sections of the journal. One recurring entry are the narrative reviews. He says that these are rarely cited so increase the denominator of the IF without moving the numerator. Despite the fact that these hurt the IF they publish them anyways because they see it as part of their mission. Not every journal editor's morals are so straight.
A different scoring system, called the H-index is used to rank authors and scientists.
I had the privilege of going to the AJKD editors meeting Kidney Week and saw first hand how IF colors a lot of what they do. Editor in chief, Dr. Levey talked about change in IF over the last decade and what happened over the last year. Then he talked about the different sections of the journal. One recurring entry are the narrative reviews. He says that these are rarely cited so increase the denominator of the IF without moving the numerator. Despite the fact that these hurt the IF they publish them anyways because they see it as part of their mission. Not every journal editor's morals are so straight.
A different scoring system, called the H-index is used to rank authors and scientists.
Brent Thoma at Academic Life in Emergency Medicine has published a ranking system for blogs and online education. This has sparked an active and colorful debate, here and here. In terms of why he did it, and he said (paraphrasing) "A scoring system will come to open access medical education content. I would like it to evolve out of the community rather than have it imposed on the community. So let's build our own score."
Brent is an ER doc and all of the blogs he ranked were of interest to ER doctors. However he published his scoring algorithm and I used it to score PBFluids, Renal Fellow Network, Nephron Power, and eAJKD.
- PBFluids edged RFN with 3.02, good for 19th place among the 60 blogs he ranked.
- Renal Fellow Network came in right below PBF with a 2.94, good enough to tie for 20th on the list.
- eAJKD, scores 2.86, good for 21st place.
- Nephron Power had 2.18, good for 36th place.
None of those three had any Google+ presence and NephronPower was hurt by the lack of a Facebook page and minimal footprint on Twitter.
You can view my spreadsheet here. Please add your website if you would like.
- Alexa is the website rank according to Alexa.com. Mine was 4,589,000. Enter the rank divided by 1,000. This is a measure of traffic.
- Page Rank is google's measure of quality by its analysis of incoming links (and likely a bunch more secret ingredients). You can find out a sites page rank here.
- Twitter is the number of followers of the principle author.
- Facebook is the number of likes for the blog on its facebook page.
- Google+ is some metric around this failed social media site.
Here are how the three sites compared:
Everyone had the same page rank of 4 except NephronPower. Alexa traffic was close, but eAJKD had the highest traffic with RFN in second. PBFluids took first based on the strength of my Twitter following.
This is very interesting to me. I have been thinking about ways to measure blog quality as part of a follow-up study from my poster presentation at Kidney Week. I tracked posts/month to measure productivity but I had no way to assess if the posts were any good. The SM-i seems like a reasonable stab at answering this question, at least at the blog/author level.
Some thoughts: purchasing twitter followers and Facebook likes is a thing and a real index should be resistant to that type of gaming. Additionally, Youtube is a very important educational channel, Nephrology on Demand is just killing it by adding educational content to YouTube, and has over 1,500 subscribers. This should be captured in the SM-i.
Saturday, November 23, 2013
Joint Commission confiscating your coffee? Time to go outlaw.
Ever had your coffee confiscated in the name of the Joint Commission.
Did you know that the JC has no standards regarding food and drink in patient care areas?
From now on, I'm going outlaw.
The JCAHO Outlaw badge is now part of the Nephrology Merit Badge collection. (PDF | Pages)
Did you know that the JC has no standards regarding food and drink in patient care areas?
 |
| From the Joint Commission's website. |
The JCAHO Outlaw badge is now part of the Nephrology Merit Badge collection. (PDF | Pages)
Thursday, November 21, 2013
UKidney's library of high impact articles
Ukidney has just posted a new page on their website. It is a library of high impact nephrology research articles. Other sites have attempted to do this but this one is particularly well done. The lead-in describes it as a growing library of "key high-impact articles." They are a little vague on the goal here but I hope they keep the list of articles small and tight. The lazy thing to do would be to include every seemingly important paper with little curation. The most value is added by maintaining editorial vigor and declaring a limited set of articles as the most important. I'd even put a number a number on it. Keep it limited to the 50 most important articles in nephrology. I like 50 because that is a number that a motivated fellow could read in a week.
A good decision in generating the list of articles was not adding the classic articles of nephrology. I was hard pressed to find an article older than 15 years. It would be easy to add Bartter and Shwartz's original description of SIADH or Thurau's brilliant essay on acute renal success but those are lessons taught in text books. We read the medical literature to find what is new and the contributors at UKidney wisely limited their list to newer data. A second, separate project might be to find the 50 most important studies in nephrology, even if they don't affect modern nephrology much, but that should not contaminate this mission.
One area that could be improved would be the addition of a 2-3 sentence description of why the contributors feel this article deserves a place in the library. Think of it as the plaque next to the picture in Cooperstown. That kind of meta-data can be so important in putting articles in scientific perspective and it would also give the organizers a venue to justify why each article is important.
To paraphrase Alan Kay, UKidney's article collection is the first one good enough to criticize.
The site is organized by 9 icons showing the different sections.
I am going to go through it section by section and describe what changes I would make. My decisions are only that, my decisions. There is no right or wrong here, I am just giving my angle.
The first section is anemia. This section has four articles, all of them negative trips of epo: Normalization of hematocrit, CHOIR, CREATE and TREAT. These clearly are the most important articles in the fall of ESAs we have witnessed in the past decade, but they are not all equal. Given the relative size of the trials, this list could be limited to the Beserab trial in hemodialysis and TREAT in CKD.
Also the lack of iron could be corrected by inclusion of DRIVE.
Bone and mineral metabolism
This section reads like a Genzyme (Sorry, I mean Sanofi) reading list. They included the original article on calcium x phos product and two separate articles on binders and coronary calcification. As appealing and intuitive as coronary calcification is, it is an intermediate end-point that is not validated to correlate with patient outcomes. This view on binders seems unbalanced especially given the conspicuous absence of the negative mortality trial of sevelamer (DCOR). Additionally all of the other dimensions of Calcium, phosphorous and PTH are ignored. Where is EVOLVE and Teng's retrospective trial of dialysis survival with and without active vitamin D therapy. Also where is Wolfe's FGF-23 and dialysis mortality study? This section needs a lot more attention. Remove the retrospective and old in order to widen the scope of the section.
Diabetes
The Diabetes page is nearly perfect. One quibbles, I would add the long term follow-up of the DCCT trial because that introduced the concept of metabolic memory and changed the original study from one that affected a questionable intermediate endpoint, microalbuminuria, and replaced it with doubling of serum creatinine.
Fluid and Electrolytes
The fluid and electrolyte page, a subject near and dear to my heart (in case you hadn't noticed), needs work and I mean a lot of work. Right now it has a case report about a disease you will never see, syndrome of inappropriate diuresis, and a collection of review papers. All of the review papers are excellent but it is hard to consider any review article a high impact article. High impact articles for this section to consider include:
- Safe Study
- Low chloride fluids trial
- Pick which recent article blasting colloids that you want
- Oxoproline / Pyroglutamic acidosis
- Meta-analysis on sodium bicarbonate in DKA (this may be the wrong link, I thought there was a Cochrane Review, that I couldn't find it)
- Case report of CPM with potassium replacement alone from AJKD
- SALT study on tolvaptan (See, we can do big RCTs on electrolytes, all we need is an expensive drug to pay for it!)
The next section is general nephrology and UKidney wisely created sub-sections to break up the 37 articles in this section. They should add a cystic disease section and a CKD section.
Lipids
Nailed it.
Acute Kidney Injury
The AKI section leads off with a couple of review articles. See above. Then it has a review and meta analysis of NGAL. This can't be high impact because the test is readily available and no one knows what to do with the data anyways. This article seems out of place in this list. The the ATN trial takes it rightful place on the list. Strangely, right after that is a meta-analysis that recommends the high dose that was disproven in the ATN trial. I say flush Panu's article. Bringing up the rear is a meta-analysis showing that iHD and CVVH can both be used in ARF. Fine.
Things that are missing from the AKI section is the big meta analysis on renal dose dopamine has a place here. I would also include some of the data from CABG that show increased mortality from minimal increases in creatinine. Also the recently completed CORONARY study along with some of the previous work linking AKI to CKD should be included.
AJKD Core curriculum
No complaints. Clever addition.
Critical Care Nephrology
This looks like a retread from AKI. Only one additional article, a Cochrane Review of IHD versus CVVH. They should collapse these two headings into one.
Glomerulonephritis
I must admit I am not as up to date on the literature in this sub-field as I should be. But overall it looks like they have too many articles on MMF for lupus (3 of the 8 articles). They have nothing on membranous or FSGS. This looks like a half-baked section. I will give them a pass as they work on filling the library.
Hemodialysis
The next major section is hemodialysis. There are a lot of articles on AKI in this section. Though we treat AKI with dialysis, I think this section should be left to chronic dialysis and include PD with HD.
In terms of missing studies. I would add the Aggrenox study on access patency. They should include some of Agarwal's work on blood pressure in dialysis patients, they should include the IDEAL study, one of the most important dialysis studies in the last decade. Another important study was Tamura's study on the deterioration of functional status with dialysis.
Hypertension
The hypertension section seems light to me. It is filled with a number of trials of different drugs. It is missing ALLHAT and AASK but more importantly it is missing aspects of hypertension beyond what drug and what blood pressure target. They should include some Symplicity data, and at least one trial on the importance of ambulatory / home blood pressure monitoring. They should include the Cochrane Review of Pharmacotherapy for mild hypertension and the DASH diet RCT.
The Peritoneal dialysis
The Peritoneal dialysis section is tiny. It makes me think that the PD and HD sections should be combined and form a new section called chronic dialysis. Two additionals would be PD for acute renal failure sepsis (don't do it) and an article on PD First.
Transplantation
I am not as familiar with this literature as I should be and I honestly don't know what should be on the list. Though I think that the CJASN study on recurrence of primary disease after transplant should be included.
Monday, November 18, 2013
All the tweets of #KidneyWk13
@kidney_boy Brilliant summary of Renal Week activity via Twitter. Particularly good way for us non-attendees to keep in touch. @ASNKidney
— Dr Damian Fogarty (@DamianFog) November 18, 2013
There was an incredible amount of twitter activity at ASN Kidney Week 13. I have downloaded the entire transcript and stitched the 9 pages into a single document for your downloading pleasure. It is 478 pages and 68,000 words long.
If that is not long enough for you could check out the hashtag misspelling at #kidneykw13 or the expanded #kidneyweek or in case you didn't want any confusion with the epic 1913 ASN Kidney Week: #KidneyWk2013.
My post on patient empowerment for World Diabetes Day
I was given the opportunity to post at the Accountable Care Organization Blog run by Dr. Robert Provenzano. I wrote about patient empowerment in diabetes and how some of those lessons could be used to improve CKD care. Take a look.
This never would've happened if the AHA/AAC had put Andrew Levey in charge of their risk calculator
Two items of note struck me this morning. The first was the fumble with the new cholesterol recommendations. The guidelines have been taking heat largely because they dramatically expand usage of statins in populations with that receive little to no benefit front he drug.
The last thing the new recommendations needed was a headline in the paper of record showing that their calculator over estimated 10 year cardiac risk by a mere 75 to 150 percent:
It is interesting that this story is just getting legs today. The alarm was raised on the day the guidelines were released. From Medscape:
 In the end, I think we can agree that the cardiologists should leave the equations and formulas to the nephrologists, namely Andrew Levey. Hence the second item of note. Andrew Levey won the prestigious Belding Scribner Award. Levey is best known for creating the MDRD eGFR estimating formula and its successor the CKD-Epi formula.
In the end, I think we can agree that the cardiologists should leave the equations and formulas to the nephrologists, namely Andrew Levey. Hence the second item of note. Andrew Levey won the prestigious Belding Scribner Award. Levey is best known for creating the MDRD eGFR estimating formula and its successor the CKD-Epi formula.
I have gotten to know him as he is the editor and chief AJKD and spearheaded the creation of eAJKD. During every one of my encounters with him he has been humble, witty and friendly. During his Scibner acceptance speech he revealed that he considers his greatest moment in nephrology to be donating a kidney to his wife.
What a mensch.
There is a great interview with Andre Levey at eAJKD today. Take a moment and read it.
 |
| TheNNT is one of my favorite websites for patient discussions. |
This week, after they saw the guidelines and the calculator, Dr. Ridker and Dr. Cook evaluated it using three large studies that involved thousands of people and continued for at least a decade. They knew the subjects’ characteristics at the start — their ages, whether they smoked, their cholesterol levels, their blood pressures. Then they asked how many had heart attacks or strokes in the next 10 years and how many would the risk calculator predict.
That's a big miss and I think it threatens to be a big mess if people lose confidence in our cardiology experts and their guidelines.The answer was that the calculator overpredicted risk by 75 to 150 percent, depending on the population. A man whose risk was 4 percent, for example, might show up as having an 8 percent risk. With a 4 percent risk, he would not warrant treatment — the guidelines that say treatment is advised for those with at least a 7.5 percent risk and that treatment can be considered for those whose risk is 5 percent.
Look what that risk calculator did to that Church!
It is interesting that this story is just getting legs today. The alarm was raised on the day the guidelines were released. From Medscape:
To heartwire , Dr Roger Blumenthal (Johns Hopkins Medical Institute, Baltimore, MD), who was not part of the writing committee, said he agreed with 90% of the information in the new guidelines. "To put that in perspective, I probably only agree with my wife 85% of the time," he said.
Namely, he is a little troubled by the new atherosclerotic risk score. Derived from FHS, ARIC, CARDIA, and CHS, it hasn't performed all that well when applied to other cohorts, such as the Multiethnic Study of Atherosclerosis (MESA) and Reasons for Geographic and Racial Differences in Stroke (REGARDS) study, he said. The risk score does not take into account family history of premature cardiovascular disease, triglycerides, waist circumference, body-mass index, lifestyle habits, and smoking history.
"In my mind, we're putting a lot of faith in this risk score," said Blumenthal. "We're probably going to be treating many more people, especially many more ethnic minorities, who get above this 7.5% threshold."
 In the end, I think we can agree that the cardiologists should leave the equations and formulas to the nephrologists, namely Andrew Levey. Hence the second item of note. Andrew Levey won the prestigious Belding Scribner Award. Levey is best known for creating the MDRD eGFR estimating formula and its successor the CKD-Epi formula.
In the end, I think we can agree that the cardiologists should leave the equations and formulas to the nephrologists, namely Andrew Levey. Hence the second item of note. Andrew Levey won the prestigious Belding Scribner Award. Levey is best known for creating the MDRD eGFR estimating formula and its successor the CKD-Epi formula.I have gotten to know him as he is the editor and chief AJKD and spearheaded the creation of eAJKD. During every one of my encounters with him he has been humble, witty and friendly. During his Scibner acceptance speech he revealed that he considers his greatest moment in nephrology to be donating a kidney to his wife.
Levey donated a kidney to his wife. TLA. #kidneywk13
— Joel Topf (@kidney_boy) November 9, 2013
What a mensch.
There is a great interview with Andre Levey at eAJKD today. Take a moment and read it.
Thursday, November 14, 2013
My Best Kidney Week
Every year that I go to Kidney Week it seems to get better. I had a wonderful time at this years Kidney Week in Atlanta primarily because it was full of new experiences and connections.
One of the highlights was getting the honor of introducing the KDIGO Mobile app. This iPad only app contains all of the KDIGO guidelines and support documents. The Chair of the implementation committee, Yusuke Tsukamoto, described me as KDIGO's Steve Jobs. I can't imagine a higher compliment.
The app is a great way to read the guidelines. We feel these are our first steps and we are excited to push the app forward. You can download it for free from the iPad App Store. Getting a chance to work with the KDIGO folks on this project has literally been one of the highlights of my career.
I aggressively live tweeted every session I attended. This is a huge 180° U-turn for me. See this post from last August where I blast the entire practice. Hobgoblin of little minds and all. Well all that tweeting resulted in me being the largest influencer of Kidney Week 2013.
The middle column is just the number of tweets by different individuals. I would like to call attention to three new tweeters. I maybe wrong, but I believe @rednephron, @ThePeanutKidney and @KatieKwonMD were all tweeting their first KidneyWeek. It is great to see new tweeters, especially ones who are so good at their craft. Welcome to the community.
One of the highlights was getting the honor of introducing the KDIGO Mobile app. This iPad only app contains all of the KDIGO guidelines and support documents. The Chair of the implementation committee, Yusuke Tsukamoto, described me as KDIGO's Steve Jobs. I can't imagine a higher compliment.
Just got my hair cut. Asked for the full Steve Jobs. What do ya think? http://t.co/DT45QxOa
— Joel Topf (@kidney_boy) September 16, 2011
The app is a great way to read the guidelines. We feel these are our first steps and we are excited to push the app forward. You can download it for free from the iPad App Store. Getting a chance to work with the KDIGO folks on this project has literally been one of the highlights of my career.
I aggressively live tweeted every session I attended. This is a huge 180° U-turn for me. See this post from last August where I blast the entire practice. Hobgoblin of little minds and all. Well all that tweeting resulted in me being the largest influencer of Kidney Week 2013.
 |
| That will be the only time I will ever be listed ahead of the New England Journal of Medicine. |
Impressions is number of tweets times number of followers. Mentions is the number of tweets where an individual is mentioned along with the hashtag #KidneyWk13. To me this is the most important metric, since it indicates tweets that are generating interactions, through retweets and replies.
Pushing the twitter at #kidneywk13 pic.twitter.com/BWNoNKeMdR
— Joel Topf (@kidney_boy) November 6, 2013
The summary statistics show increasing twitter use at the meeting. Here are 2013's numbers compared to 2011 and 2012:
Tweets and participants are rising.
I also did a fair amount of blogging:
And I was invited to present my NephMadness abstract during an oral presentation. What an honor. I don't have any photos but I did a screen cast of the presentation:
Nephmadness Unplugged is available here.
Nephmadness Unplugged is available here.
Tuesday, November 12, 2013
So now that we know that ZS-9 lower potassium, how much do you think it will cost?
One of the only positive trials at Kidney Week's Late Breaking Trials Session was ZS-9, a novel potassium binding crystal that hopes to take the place of Kayexalate (Sodium Polysterene) in the hyperkalemic cocktail.
I wrote about the trial for eAJKD and was quoted in an article for MedPage Today.
Here is the skinny:
I wrote about the trial for eAJKD and was quoted in an article for MedPage Today.
Here is the skinny:
- ZS-9 uses potassium specific pores to bind potassium rather than a cation exchange resin. It is highly specific for potassium, especially when compared to SPS.
- In the phase 2 trial 10 grams lowered the potassium by 0.04 mEq/L per hour
- In the phase 3 (753 patients!) trial the 10 gram dose lowered the potassium 0.78 mEq/L in 14 hours
- No diarrhea
- GI side effects were equal in the placebo and drug arms
I suspect this drug is on a smooth glide path for approval. My question is how expensive will it be? My guess is $200 per 10 grams. Please add your guess or over-under bet to the comments or @kidney_boy on Twitter.
Monday, November 4, 2013
Tolvaptan, ADPKD, and lack of vision at the FDA
About a year ago the ASN Kidney Week hosted the most exciting Late Breaking Trial Session I have attended. The session included the first public disclosure of the EVOLVE trial and the incredibly exciting results from the TEMPO 3:4 trial. I blogged about this for eAJKD and felt then, and still feel now, that this was an incredible breakthrough for nephrology. Unfortunately that announcement may ultimately be the high point for tolvaptan. In April the company acknowledged previously unsuspected liver toxicity. Then in August the FDA denied the application for an indication for ADPKD. Tolvaptan is still approved for hyponatremia and doctors can always prescribe the drug off label but the drug is so expensive I think few patients will be able to get it approved by their insurance companies leaving them to face the $273,000/year bill alone. I always suspected that Otsuka would change the price of the drug when they got a second indication for the drug that changed it from a short-term, in-house drug to a chronic out-patient indication. We may never know.
This is the second significant set back for tolvaptan. Otsuka had investigated it for heart failure with the flawed (in my mind) Everest Trial. Tolvaptan never sought an indication for heart failure because their trial was negative. This is what makes the FDA’s decision so upsetting, the TEMPO trial was positive, the drug met its primary end point (P=0.001), it slowed cyst growth. The secondary end-point, and more clinically relevant end-point, of decreased change in GFR was also positive (P=0.001).
Side-note about the choice of change in kidney volume rather than change in GFR for the primary end-point: This was used because ADPKD is such a slowly progressive disease that investigators feared if a treatment was required to slow the change in GFR, it would be prohibitively expensive and slow to evaluate therapies. The change in kidney volume was adopted by the ADPKD research community as an acceptable intermediate end-point after observational imaging studies found a tight relationship between change in kidney volume and renal prognosis.
When I heard about the denial I figured the liver toxicity must have much more severe than I suspected. That turns out not to be the case. Bill Brazell (Bill is a former board member of the PKD Foundation and reader of PBFluids, who was at the FDA hearing) described the liver toxicity founding the TEMPO trial:
The thing that frustrates me the most is that there are no other proven treatments. We have nothing to offer our patients that works, the only effective therapy was denied approval. Silly FDA what do they expect these patients to do?
I hope Otsuka stays the course, provides additional outcomes data so we can get this approved and I hope the FDA opens it's eyes and begins to see that in the absence of perfect we should accept good.
The article I linked to by Bill Brazell is an excellent discussion of the same issue from a patient perspective. Read it.
This is the second significant set back for tolvaptan. Otsuka had investigated it for heart failure with the flawed (in my mind) Everest Trial. Tolvaptan never sought an indication for heart failure because their trial was negative. This is what makes the FDA’s decision so upsetting, the TEMPO trial was positive, the drug met its primary end point (P=0.001), it slowed cyst growth. The secondary end-point, and more clinically relevant end-point, of decreased change in GFR was also positive (P=0.001).
Over a 3-year period, the increase in total kidney volume in the tolvaptan group was 2.8% per year (95% confidence interval [CI], 2.5 to 3.1), versus 5.5% per year in the placebo group (95% CI, 5.1 to 6.0; P=0.001). The composite end point favored tolvaptan over placebo (44 vs. 50 events per 100 follow-up-years, P=0.01), with lower rates of worsening kidney function (2 vs. 5 events per 100 person-years of follow-up, P=0.001) and kidney pain (5 vs. 7 events per 100 person-years of follow-up, P=0.007). Tolvaptan was associated with a slower decline in kidney function (reciprocal of the serum creatinine level, -2.61 [mg per milliliter](-1) per year vs. -3.81 [mg per milliliter](-1) per year; P=0.001). There were fewer ADPKD-related adverse events in the tolvaptan group but more events related to aquaresis (excretion of electrolyte-free water) and hepatic adverse events unrelated to ADPKD, contributing to a higher discontinuation rate (23%, vs. 14% in the placebo group).
Side-note about the choice of change in kidney volume rather than change in GFR for the primary end-point: This was used because ADPKD is such a slowly progressive disease that investigators feared if a treatment was required to slow the change in GFR, it would be prohibitively expensive and slow to evaluate therapies. The change in kidney volume was adopted by the ADPKD research community as an acceptable intermediate end-point after observational imaging studies found a tight relationship between change in kidney volume and renal prognosis.
When I heard about the denial I figured the liver toxicity must have much more severe than I suspected. That turns out not to be the case. Bill Brazell (Bill is a former board member of the PKD Foundation and reader of PBFluids, who was at the FDA hearing) described the liver toxicity founding the TEMPO trial:
A small number of patients experienced potentially important elevations of liver enzymes (4.9 percent, compared to 1.2 percent of those who took a placebo), but the panel focused for hours on the simultaneous elevations in both liver-enzyme levels and bilirubin that occurred in just three patients out of the 860 who took the drug. In all three, the elevations occurred within 18 months. After those patients stopped taking tolvaptan, their levels returned to normal. No one suffered permanent damageCertainly tolerable to my eyes, especially considering the health implications of dialysis and kidney transplant.
The thing that frustrates me the most is that there are no other proven treatments. We have nothing to offer our patients that works, the only effective therapy was denied approval. Silly FDA what do they expect these patients to do?
I hope Otsuka stays the course, provides additional outcomes data so we can get this approved and I hope the FDA opens it's eyes and begins to see that in the absence of perfect we should accept good.
The article I linked to by Bill Brazell is an excellent discussion of the same issue from a patient perspective. Read it.
Sunday, October 20, 2013
Calling all Apple nerds! See if you can predict what will be announced...Updated!
Apple already announced new iPhones and has a media event scheduled for October 22nd. If you think you have some Apple game see if you can predict the announcements. Go here to fill out your entry. Voting ends Tuesday morning.
UPDATE
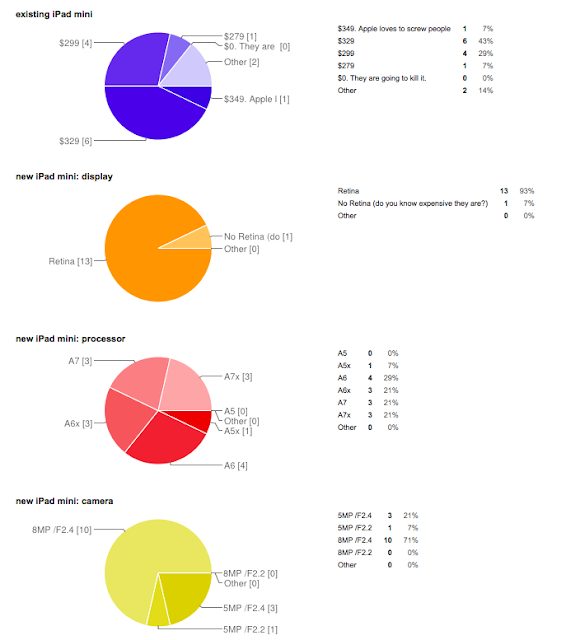
The winner is...Milos who crushed the competition with a score of 40! He correctly nailed the price of the price of the two iPad minis, the camera specs of both new iPads, almost perfectly nailed the specs of the iPad Air (only blemish predicting an A7x rather than the A7). But where he really lapped the competition was predicting that the program would ignore the iPods and Apple TV. Strong work!
Second place was Steven with 30 points. I tied for fourth with Mhallang at 28 points. Thanks for playing.
You can see all of the submissions here.
Kidney Week Approaches!
This year for the first time (that I am aware of) Kidney Week is in Atlanta.
I was lucky enough to get both an abstract and oral presentation accepted. Both are on social media.
The Oral presentation is on the lessons from NephMadness. I will be speaking at 5:30 on Friday, November 8, in Room 409. The session is titled Nephrology Education and Research and runs from 4:30 to 6:30.
The poster presentation is on Thursday November 7 from 10 to 12. The poster is a review of productivity, longevity and consistency in nephrology blogs. It is poster number TH-PO880.
I would love to meet any readers of the blog, so stop by. Also if you post your abstract/poster information as a comment to this post I will make every effort to visit your poster and review it on PBFluids.com from a design and nephrology perspective. Think of it as The Blogger Eye for the Scientist Guy.
See you in Atlanta!
I was lucky enough to get both an abstract and oral presentation accepted. Both are on social media.
The Oral presentation is on the lessons from NephMadness. I will be speaking at 5:30 on Friday, November 8, in Room 409. The session is titled Nephrology Education and Research and runs from 4:30 to 6:30.
See you in Atlanta!
Friday, October 18, 2013
Journal Club Wasteland
Nephrology and social media has some notable successes:
- The Kidney Group has charted a unique course with Facebook by breaking the so called rules of social media in medicine. The Kidney Group regularly highlights individual patients, and the private lives of the doctors, nurses and families of the people who work at this private practice. They have a unique voice and engage with a community.
- Renal Fellow Network has created a website that that is remarkable for its scope, longevity and quality. They are the only nephrology website that has mastered succession, an essential element of long term success.
- eAJKD and NephronPower, the twin brain products of Kenar Jhaveri have demonstrated remarkable creativity in making nephrology more approachable and interesting with word games, detective stories and NephMadness.
- Twitter has increasingly been an amazing social media destination for intelligent nephrology discussion. Two particular tweeterari standout:
- Ed Lerma is doing great work with his #NephPearls project.
- And Christos Argyropoulos (feels like I should get some kind of reward for spelling that correctly) is defining nephrology on twitter by his frequent engagement and general cleverness.
- The Ajay Singh and The Kidney Doctor has had tremendous success, it is fascinating having an A-level scientist join the social media ghetto. His productivity and gravitas provides a unique voice.
- This is by no means a comprehensive list. Notable additions which immediatly come to mind include Tejas Desai, Mathew Sparks, Jordan Weinstein, Mahesh Krishnan and I am sure I have forgotten too many others. (Please post your umbrage in the comments)
This project had a simple, straight-forward goal. Take an article from CJASN every month or so. Highlight it by giving both it, and the editorial away. Make the author available for some questions. Create an interesting online discussion of the article. Create, a national or even international nephrology journal club.
Here is a list of the discussions for this month's article on Mediterranean Diets (October):
Here are the discussions for the initiation of RRT in CKD (September):
The April '13 article on marajuana has no comments! If you can't get the internet to comment on weed, you have a problem (the likely issue here was posting article on 4/22. Total missed opportunity, 4/20 was just sitting there waiting for a post on dope).
March and February, no comments.
You get the idea. Here is the data in aggregate
The January '13 post has 3 comments, unfortunately they have nothing to do with The effects of hemodiafiltration on quality of life:
This is a failed project. Why did it fail?
I can't get past the lock on the home screen of the project
What a way to turn people away. The site literally has a padlock on the front door. The idea of an online journal club is that the wisdom of the crowd can shine through. With enough eyes and mouths all aspects of an article can be turned over and vetted. By establishing this barrier to entry they limitted participation and turned people away. Most internet forums allow everyone to view discussions and only require logging in to comment. I can't think of any reason to prevent people from browsing the discussion without first logging in.
The discussions are labelled not by first name but by e-mail address. So you know FunGuy87@yahoo.com is going to be reluctant to leave a comment due to profound e-mail address embarrassment. Though I have to say I got a bit of a thrill seeing the e-mail addresses of George Bakris, John Asplin, Dave Goldfarb and other rock stars of nephrology revealed in their forum posts. In my opinion people want some degree of anonymity. CJASN did not allow that. But compared to other mistakes, I think this one is unimportant. Fixing this will not fix the Journal Club.
The funny thing is that the head of the site gets it. David Goldfarb has this blurb under latest news (note: if the link is from May 2012, time to change it from latest news):
This link is to an interesting editorial from the NYT about the value of free access and opening yourself up to critique by the public. I wonder if that blurb was Goldfarb's way of nudging the gatekeepers that put the lock on the door?
But the reason CJASN Journal Club failed is this is a project that depends on a community it does not have. I spoke with Dr. Goldfarb about this and he realized this and told me that he hoped to create the community with the journal club itself.
The idea of an online journal club is a good one and has been executed in multiple venues successfully. The one I am most familiar with is the Twitter Journal Club run by Natalie Silvey. Transcripts from the journal clubs can be found here and here. I spoke with Natalie about her experience over twitter. Some interesting tidbits:
- choose articles with general appeal
- you don't need to be a twitter superstar, she had only 500 followers when she launched it
- publicity helps: the project was featured in Nature(!) and a number of her friends and followers were medics who helped stoke interest. This has started an avalanche of publicity and imitators. (see here, here and here)
- The twitter format is very accessible to everyone
- Since the host had no specific authority (she was a junior attending when she started it) it radiated approachability by other people. Having an author or similar authority figure can stifle discussion with presumed expertise.
One thing that I like about the Twitter Journal Club is that it's an event. It convenes at a set time and you are either there or you miss it. You can go back afterwards to scan the transcripts but the event is done. Having people discuss things in real time makes it feel more collegial and interactive, a static forum is one derivative further away from the tradition journal club. An important part of the success of the twitter journal club is a blog that holds the transcript and the opening statement. A blog can be indexed by google so it is findable and preserved. Putting the journal club behind a password blocks this utility.
In fact, the password protection gives the impression that this knowledge is off limits, It is a rejection of one of the core principles of Medicine 2.0: openness, transparency and accepting the truth that the monopoly of information that physicians used to have no longer exists.
So where does this leave CJASN Journal Club? Dr. Goldfarb admits that they are at a cross roads and shuttering the whole site is being openly discussed. I think that unless they are willing to try a whole new strategy they should just put the thing out of its misery.
If they want to try CJASN Journal Club 2.0 I would suggest:
So where does this leave CJASN Journal Club? Dr. Goldfarb admits that they are at a cross roads and shuttering the whole site is being openly discussed. I think that unless they are willing to try a whole new strategy they should just put the thing out of its misery.
If they want to try CJASN Journal Club 2.0 I would suggest:
- No paywall, password, or mandatory e-mail collection
- Run the journal club on twitter or in a Google Hangout
- Make it an event by partnering with other nephrology social media hubs (essentially admit that you don't have a community so you will borrow RFN's or PBFluid's or NOD's for a session
- Dump the forums and
- Replace them with a blog with three or four posts per month:
- first post: introduction to the article
- second post: editorial or author interview
- third post: post for the twitter event (this could be combined with #1), include the questions that will be addressed during the chat
- Fourth post: summary after the event with the transcript and analytics from Symplur
- Allow comments on the blog to be the replacement for the forums
I allowed Dr. Goldfarb to review this before it was posted and I gave him an opportunity to respond.
Joel,
I appreciate the opportunity to read your post and reply. I have to admit that I was hoping for more participation by the nephrology community in eJC; I too envisioned something more active. We at CJASN are very receptive to this feedback, as, in truth, we have had little feedback from ASN members before your post. So we are glad that you wrote and glad to consider ways to make eJC better.
There are other measures of participation, other than the amount of discussion, that perhaps are evidence of a higher level of success than you suggest. Maybe that participation is more passive. I’m not sure what measure of “success” you are applying to some of the online activities you cite. The Renal Fellow Network is terrific but the very thoughtful posts there usually have 0 to few comments made.
Perhaps you will judge eJC as less of a wasteland if I give you some recent stats. There are 969 people subscribed who get the monthly email. In October 44 new subscribers have enrolled so we have not stopped. For September-October we have had 350 visits by 295 unique visitors, with close to 4 page views per visit. All of those sound pretty good to me.
I also have some anecdotal tales to share. My fellow’s father is a nephrologist in India: his group discusses the eJC article every month. “He loves it”, she tells me. There were a couple of formal discussions of eJC papers at King Faisal Hospital in Riyadh under the leadership of a former NYU fellow. MAYBE there are nephrology fellows choosing our article to discuss for their fellowship program’s journal club…maybe fellows are less likely now to ask their faculty to suggest an article to present.
I have thought several times that we need to do a survey of the subscribed members and figure out what activity out there is actually ongoing, because clearly I do not have the data about those sorts of activities.
One nice service that eJC, CJASN, and ASN provide is that the article is distributed free when it would otherwise remain available only to ASN members. So if it is simply a good will gesture of ASN and CJASN to the world, I don’t think that’s a bad thing.
As for “building a community”, I was inspired by Nephrol, Kim Solez’ listserv that started in the late ‘90s and remains intact and useful. I have seen it nearly every day for more than 15 years. People have been reading it and participating in case discussions all that time…it’s self-sustaining. Maybe I have to put more time into it myself, keep the conversation juiced and pertinent and engage the authors in some dialogue, but I didn't want it to be MY opportunity to chat and exchange talk with the authors, I wanted to offer that chance to the nephrologists reading out there.
As for posting anonymously as you suggest; Nephrol is not anonymous, the participants are adults and posting serious stuff, not fooling arounds. It’s like nephrologists you never heard of getting up at a microphone at the annual meeting and saying “I’m Jones, from New York, and I’d like to know what you do with calcium phosphate stones in the salivary duct”, I didn’t think it was too important to offer anonymity. There have also been discussions showing that anonymity is responsible for many of the internet’s abuses and uglier sides. But we are willing to try that.
The journal clubs that happen at a particular time on Twitter are great and novel. Everyone has a time that they can participate and a time to browse what’s online. The one from BJU on stones and soda was useful and entertaining. But how to judge the success of those events is not clear: are you satisfied if there are 5 participants? 10? As a former chief resident, I gave up counting housestaff attendance at conferences: if one person yearning for knowledge shows up and thinks about the subject, I’ll be satisfied.
On a more minor note, I see your point about the lock on the entrance being slightly off-putting…maybe an open door would be a more welcoming symbol. ASN reasonably wants everyone getting to the site to have an ASN password. It’s free and does not require ASN membership. Perhaps some shy people see the lock and feel turned away.
We’ll have further discussions about this interesting subject. I’m grateful for this opportunity to think about what we have done and what we will do. Believe me, I am much happier being a “high profile failure” than a “low profile failure”, though I’m contesting that term, and I’m looking forward to you telling me how many people read your post and my reply.
I admire the hard work you put into your website and lectures, and your intelligent critique of the nephrologic world.
Yours,
David S. Goldfarb, MD, FASN
Associate Editor and eJournal Club Editor, CJASN
Chief, Nephrology Section,
New York Harbor VA Healthcare System
Professor of Medicine & Physiology, NYU School of Medicine
Subscribe to:
Posts (Atom)